CMSC 2024
The Elliot Lewis Center attended the
Consortium of Multiple Sclerosis Centers 2024

The 2024 Annual Meeting was held in Nashville, Tennessee May 29-June 1. The Annual Meeting of the CMSC is the largest North American meeting for healthcare professionals and researchers engaged in MS care. The CMSC Annual Meeting brings these professionals together to learn from MS thought leaders about cutting edge research findings, clinical advances in the diagnosis and management of MS, and emerging care issues.
The Elliot Lewis Center team attended meetings, educational sessions and presented research posters.
This year at CMSC Fred Lublin gave an excellent lecture summarizing our current understanding of MS disease subtypes. As with everything else with MS, it’s far more complicated than we originally thought! As we understand more and more about the biology that drives relapses and progressive disease it’s become clear that our current classification of relapsing and progressive MS is outdated. New MRI techniques and biomarkers will give us a better measure of invisible disease progression.

L to R: Dr. Joshua Katz, Paige Greenawalt: Research Coordinator, Dr. Andrew Bouley,
Marissa Shackleton: Executive Director, Meg Fearey: Research Coordinator
The Elliot Lewis Center and our research department, Dragonfly Research, presented 3 posters at the meeting. Two of the posters presented were focused on our ACAPELLA data. ACAPELLA is our in-house research project, studying the real world experience with Ocrevus. We presented our year seven data, as well as our data on hypogammaglobulinemia. These posters and prior research are listed on our website at https://elliotlewisms.com/our-research/
Our new project this year studied two different premedication regimens for ocrelizumab and showed comparable tolerability and treatment related reactions. Oral cetirizine and dexamethasone led to less drowsiness and shorter chair time compared to oral diphenhydramine and IV dexamethasone. This poster was featured by NeurologyLive.
Research Presented by The Elliot Lewis Center at CMSC 2024
research is summarized below, for full posters and prior research visit: https://elliotlewisms.com/our-research/
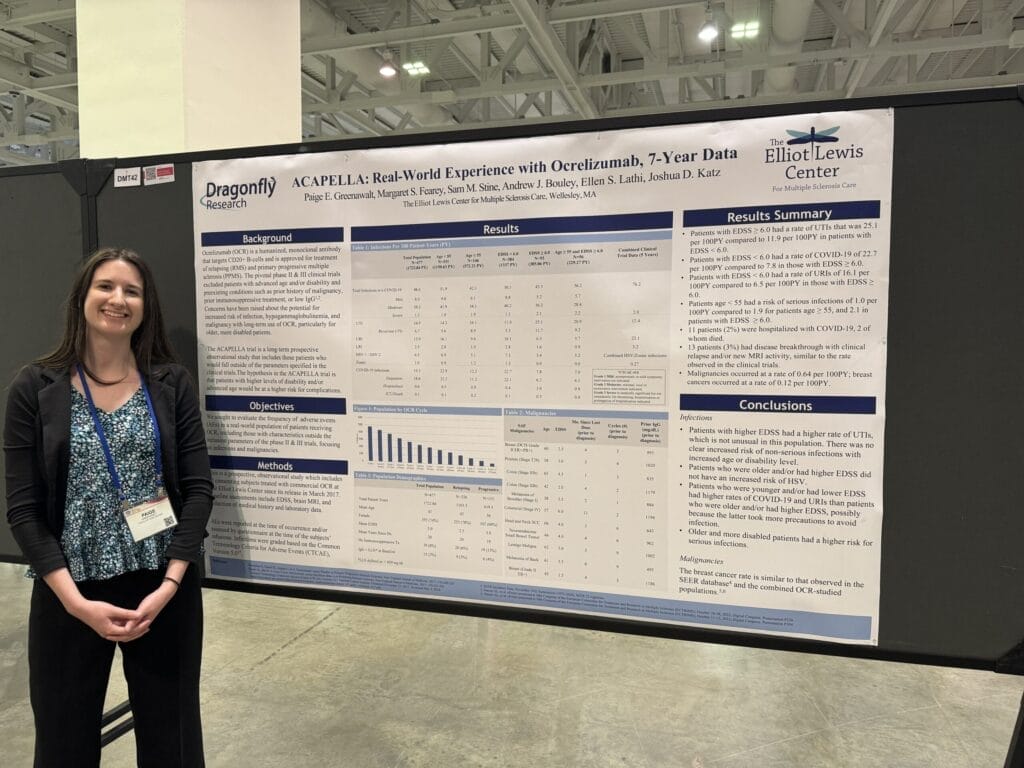
“Real-World Experience with Ocrelizumab, 7-Year Data” highlights the safety outcomes overtime of our patients treated with Ocrevus. Specifically, we included patients who were older and/or had higher disability levels, as these groups of participants were left out of the original Ocrevus clinical trials. We hypothesized that those with higher levels of disability and/or advanced age would have a greater risk of complications (infections and malignancies), but we generally did not find this to be the case. While older and/or more disabled patients had a higher rate of urinary tract infections and a slightly higher rate of serious infections, they had a similar or even lower rate of all other categories of infection than their younger/less disabled counterparts (presumably due to behavioral differences). The malignancy rate in our patient population was also similar to that of other Ocrevus-studied populations. Overall, the safety data for our patients treated with Ocrevus continues to look promising.
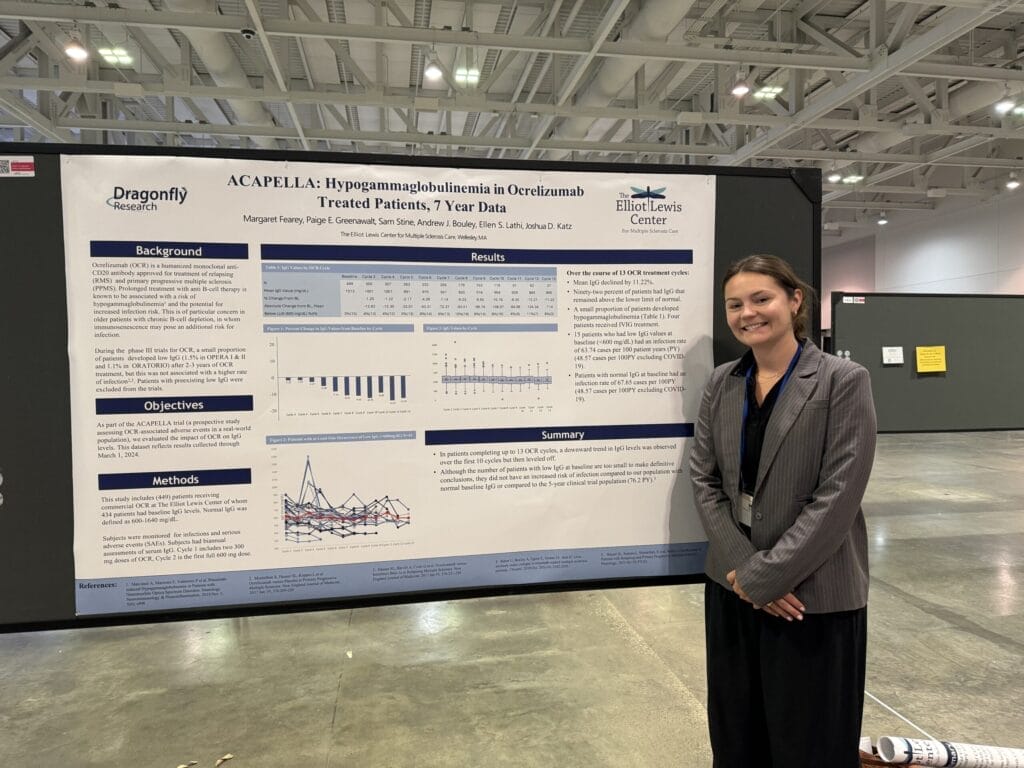
“Hypogammaglobulinemia in ocrelizumab treated patients, 7 year data”, highlights our evaluation of the impact of ocrelizumab (OCR) on IgG levels and infection rates. During the phase III trials for OCR, a small proportion of patients developed low IgG after 2-3 years of OCR treatment, but this was not associated with a higher rate of infection. Our results support the clinical trial results. Patients with a low IgG level at baseline did not display a higher infection rate than those with a normal level of IgG at baseline.
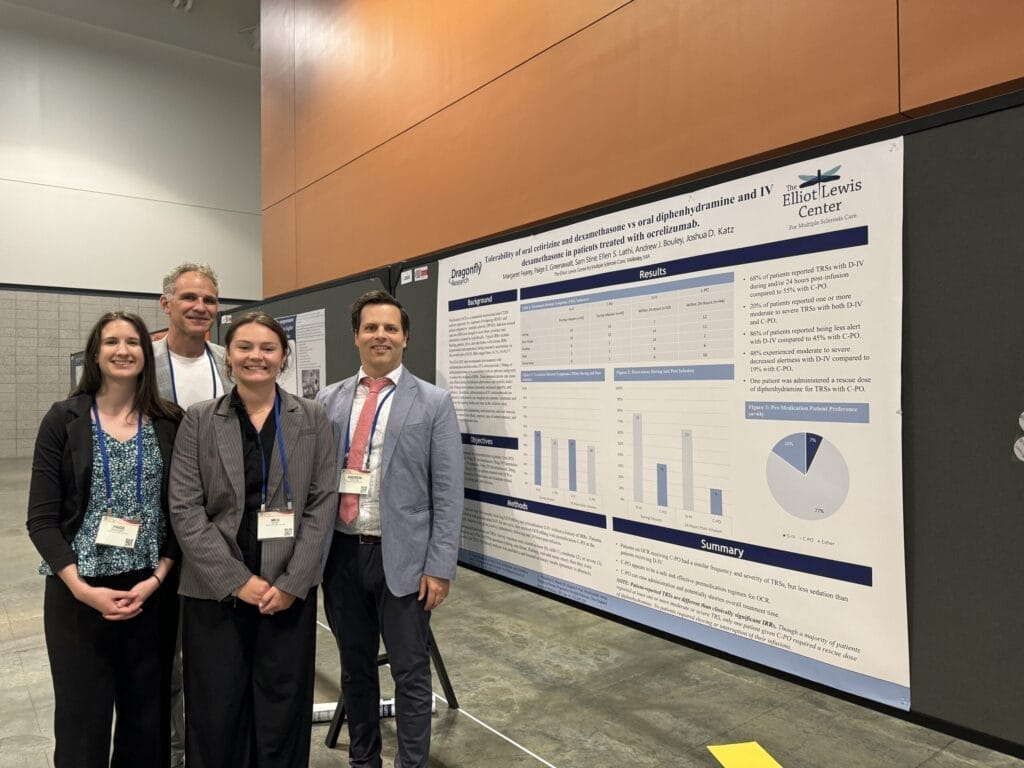
“Tolerability of oral cetirizine and dexamethasone vs oral diphenhydramine and IV dexamethasone in patients treated with ocrelizumab” highlights the comparison between two premedication regimens for patients treated with ocrelizumab (OCR). Our goal was to determine the frequency of drowsiness and treatment-related symptoms (TRSs) during and post infusion. The premedications we compared were oral (PO) diphenhydramine 50mg, IV dexamethasone 20mg, PO famotidine 20mg (D-IV) vs. PO cetirizine 10mg, PO dexamethasone 20mg, PO famotidine 20mg (C-PO). Our results from this study show that the C-PO is a safe and effective premedication regimen for OCR. This premedication alternative to D-IV also can ease administration and shorten overall treatment time.